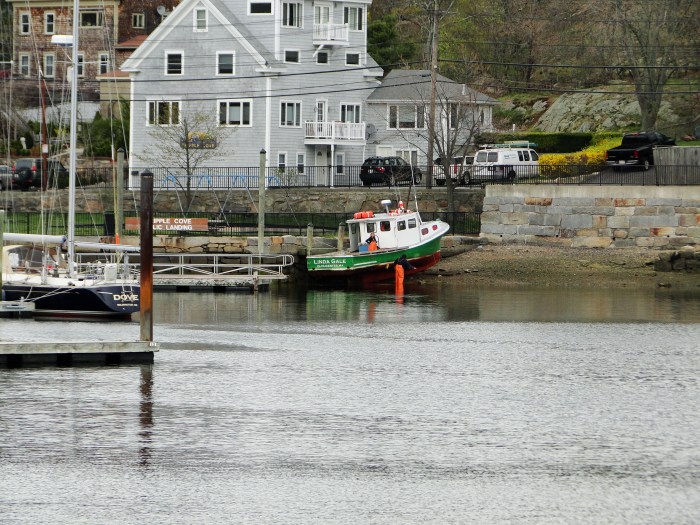Tag: Beautiful Industry
Beautiful Industry- Viking’s Office Door At The East Gloucester Marine Railways As Seen From Inside The GMG Gallery Khan Studio
Beautiful Industry- Tug Success Ends up At The Railways
Early In the afternoon the Tugboat Success cruised past the dock. In the evening she was on the blocks at The east Gloucester Marine Railways.
Rocky Neck Boats
Waiting
“Art, Rocks!” May 21, 2011 7:30pm
Video-The Gear Room At The Gloucester Marine Railway
Places In Gloucester You’ve Probably Never Been-The Gear Room At The Gloucester Marine Railway
As part of my ongoing search to bring you places in Gloucester that you probably never had access to I present you the gear room in which the motor that pulls up to 200 tons of boat out of the water at the Marine Railways at The Gloucester Maritime heritage Center resides.
Look for the video tomorrow morning
Time for a Science Lesson. There will be a Test
Time for a Science Lesson.
Just kidding! There won’t be a Test.
I’m guessing not many GMG Readers would be able to pass.
RUST!
From WIKIPEDIA;
“Oxidation of iron metal
When iron is in contact with water and oxygen, or other strong oxidants and/or acids, it rusts. If salt is present as, for example, in salt water, it tends to rust more quickly, as a result of the electro-chemical reactions. Iron metal is relatively unaffected by pure water or by dry oxygen. As with other metals, like aluminium, a tightly adhering oxide coating, a passivation layer, protects the bulk iron from further oxidation. Thus, the conversion of the passivating iron oxide layer to rust results from the combined action of two agents, usually oxygen and water. Other degrading solutions are sulfur dioxide in water and carbon dioxide in water. Under these corrosive conditions, iron hydroxide species are formed. Unlike iron oxides, the hydroxides do not adhere to the bulk metal. As they form and flake off from the surface, fresh iron is exposed, and the corrosion process continues until all of the iron is either consumed or all of the oxygen, water, carbon dioxide, or sulfur dioxide in the system are removed or consumed.[2]
Oxidation of iron metal
When iron is in contact with water and oxygen, or other strong oxidants and/or acids, it rusts. If salt is present as, for example, in salt water, it tends to rust more quickly, as a result of the electro-chemical reactions. Iron metal is relatively unaffected by pure water or by dry oxygen. As with other metals, like aluminium, a tightly adhering oxide coating, a passivation layer, protects the bulk iron from further oxidation. Thus, the conversion of the passivating iron oxide layer to rust results from the combined action of two agents, usually oxygen and water. Other degrading solutions are sulfur dioxide in water and carbon dioxide in water. Under these corrosive conditions, iron hydroxide species are formed. Unlike iron oxides, the hydroxides do not adhere to the bulk metal. As they form and flake off from the surface, fresh iron is exposed, and the corrosion process continues until all of the iron is either consumed or all of the oxygen, water, carbon dioxide, or sulfur dioxide in the system are removed or consumed.[2]“
Last of the Old Timers
Welding Days
A couple of Green Boats
The Linda Gale Again
Waiting Their Turn
SPRING CLEANING
Inside Eastern Point Light House Tour
The Captain Joe, from Captain Joe’s
Beautiful Industry Lobster Trap Buoys



lavoro di notte!
Beautiful Industry- Freshly Painted Lobster Trap Bouys